REACTIVITY OF CARBOXYLIC ACIDS
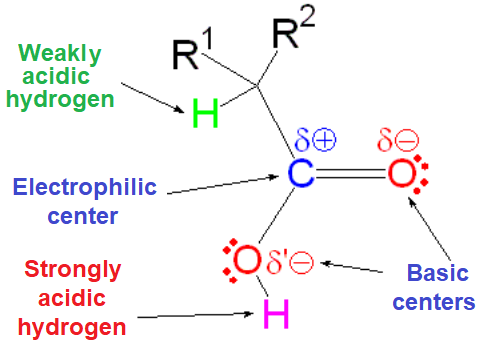
The reactivity fingerprint of a carboxylic acid is defined in the first place by the high acidity of its OH group and, in the second, by the electrophilicity of its carbonyl carbon.
IMPORTANT: The relative acidity of the alpha hydrogens to a carbonyl group is completely masked in carboxylic acids by the much higher acidity of the OH group.
The high acidity of the carboxyl group strongly interferes with any reaction carried out in basic medium.
The base is instantly neutralized by the carboxyl group and the reaction won't proceed.
Therefore, the nucleophilic attack to the carbonyl carbon of a carboxylic acid can only be performed in acidic medium.
The mechanism of the nucleophilic attack to a carboxylic acid or an acid derivative is known as addition-elimination reaction because it is made of these two steps.
Addition takes place first and elimination follows.
Addition-Elimination General Mechanism
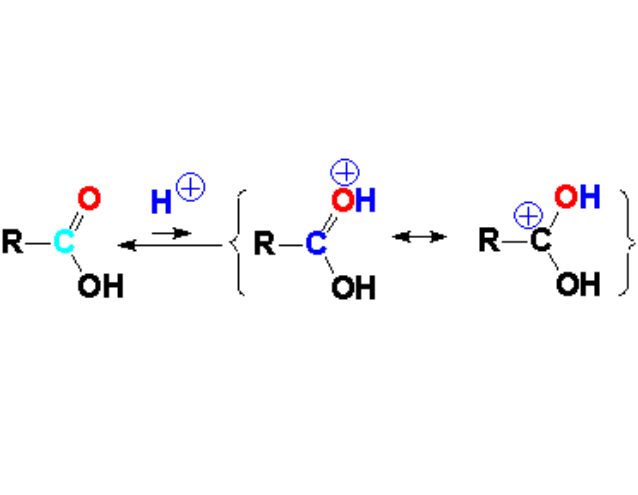
The nucleophilic attack to a carboxylic acid is performed in mineral or Lewis acidic medium, in order to avoid the OH's acidity interference and also to increase carbonyl carbon's electrophilicity.
In fact, a carboxylic acid partially protonates in acidic medium thus decreasing the electron density at the C=O carbon.
The oxygen of the C=O group is a weak base that can react with the mineral or Lewis acid. The equilibrium is shifted to the left but the few molecules that react with the external acid are much more reactive towards a nucleophile because their electron defficiency is heavily increased.
Look at the resonance forms!!!
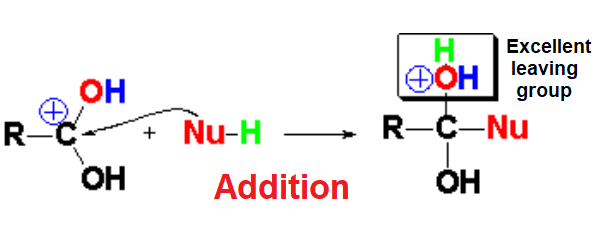
At this favorable stage of increased electrophilicity, the protonated nucleophile - we are in acidic medium! - adds to the C=O group.
Please, note that in the addition step the initially sp2 hybridized C=O carbon (trigonal planar) swaps to the sp3 hybridization (tetrahedral).
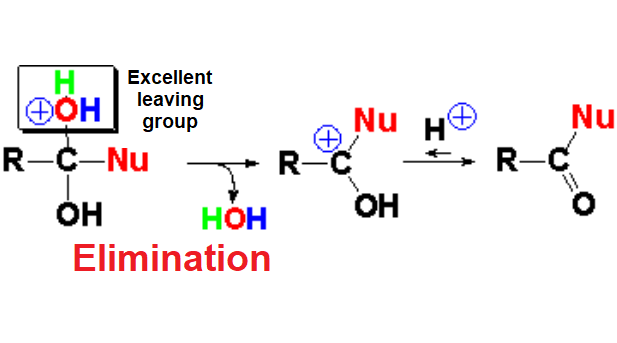
In the addition step an emergent water molecule is formed that is an excellent leaving group, leading to the carboxylic acid derivative where its initial OH has been formally replaced by the nucleophile.
In the elimination step the reacting carbon returns to its initial sp2 hybridization.
The used proton or Lewis acid is recovered and thus the needed amount of acid for this reaction is just a catalytic one.
Reactions of Carboxylic Acids