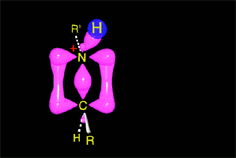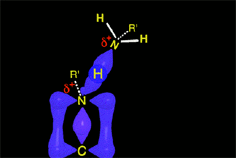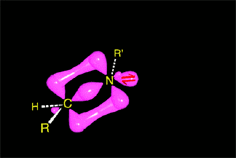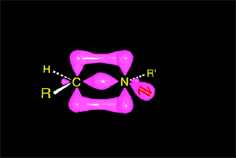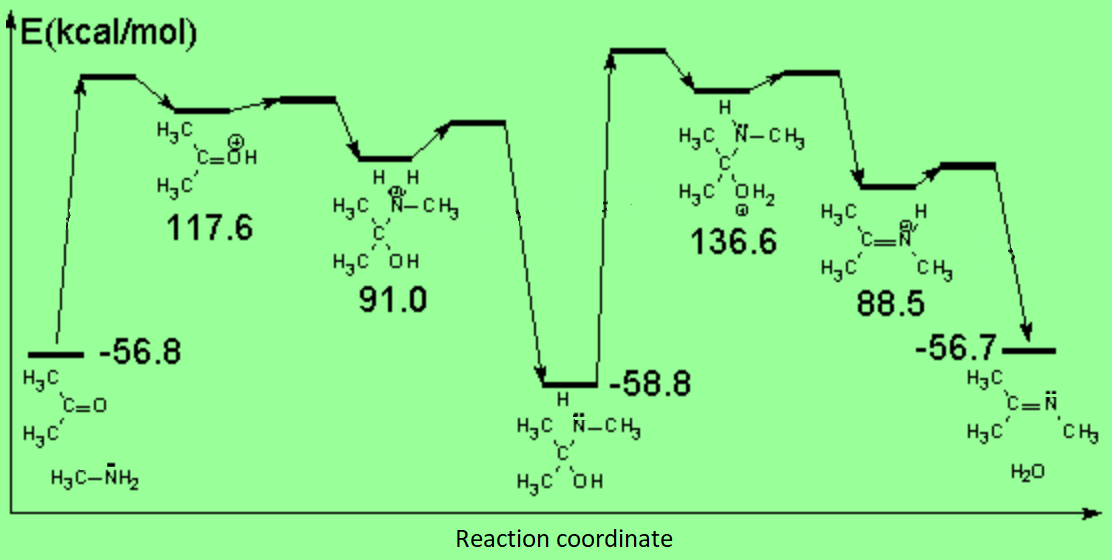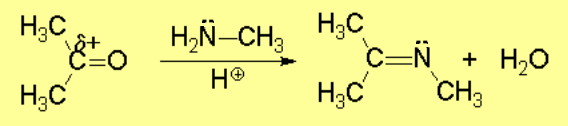
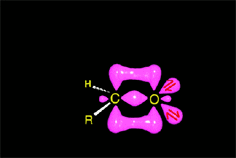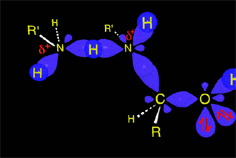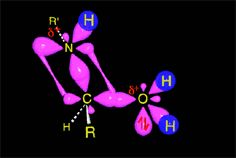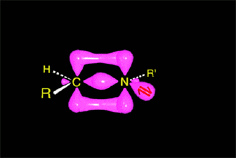
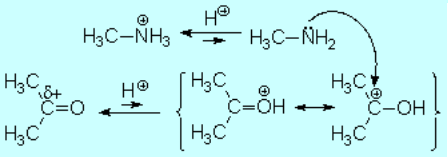
 Acid catalysis produces the protonation of some ketone molecules. These few protonated ketones have their carbon electrophilicity enhanced.
Acid catalysis produces the protonation of some ketone molecules. These few protonated ketones have their carbon electrophilicity enhanced.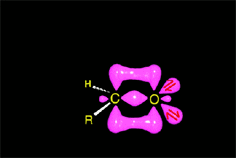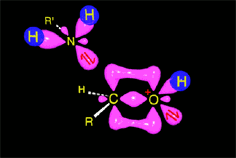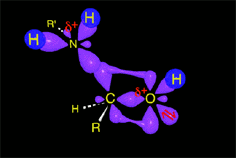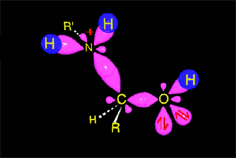
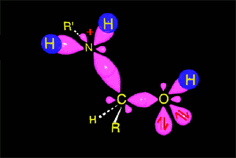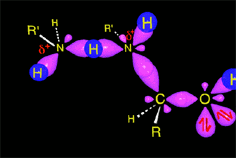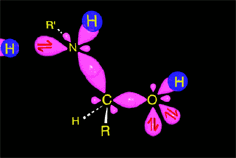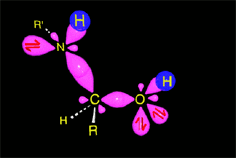
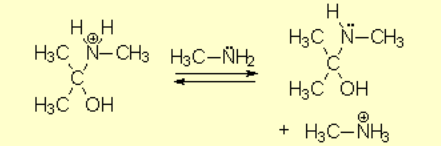 The protonated tetrahedral intermediates give up one proton to the amine molecules that didn't react yet, producing a geminal aminoalcohol (geminal = both functions bonded to the very same carbon) of high stability.
The protonated tetrahedral intermediates give up one proton to the amine molecules that didn't react yet, producing a geminal aminoalcohol (geminal = both functions bonded to the very same carbon) of high stability.
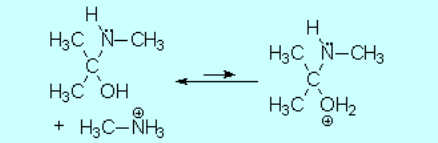 Part of the aminoalcohol molecules suffer protonation on the OH which is feebly basic, leading to an intermediate with an excellent leaving group: water.
Part of the aminoalcohol molecules suffer protonation on the OH which is feebly basic, leading to an intermediate with an excellent leaving group: water.
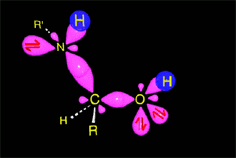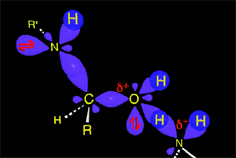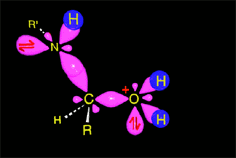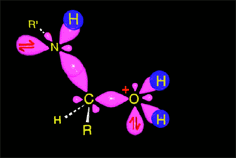
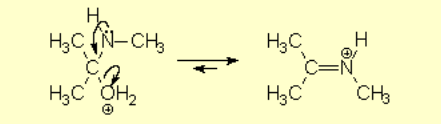 The weakened C-O bond, due to the protonation, heterolytically breaks up leading to an intermediate immonium ion.
The weakened C-O bond, due to the protonation, heterolytically breaks up leading to an intermediate immonium ion.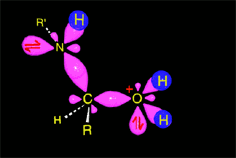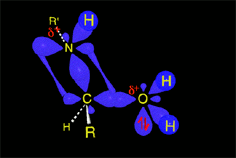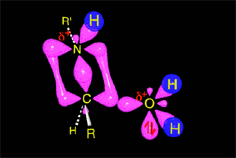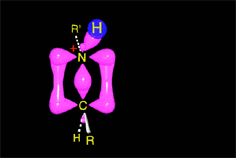
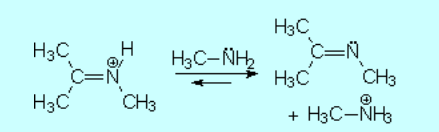 The excess of amine deprotonates the immonium salt, yielding the final imine.
The excess of amine deprotonates the immonium salt, yielding the final imine.