In this example we are solving a isomerism and stereochemical problem:
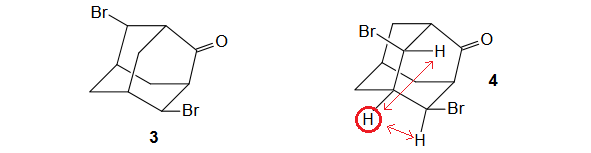
HBr catalyzes the rearrangement of bromo-oxahomoadamantanone 1. The reaction gives a unique isomer whose structure can be any of 2-5.
Which is the result?
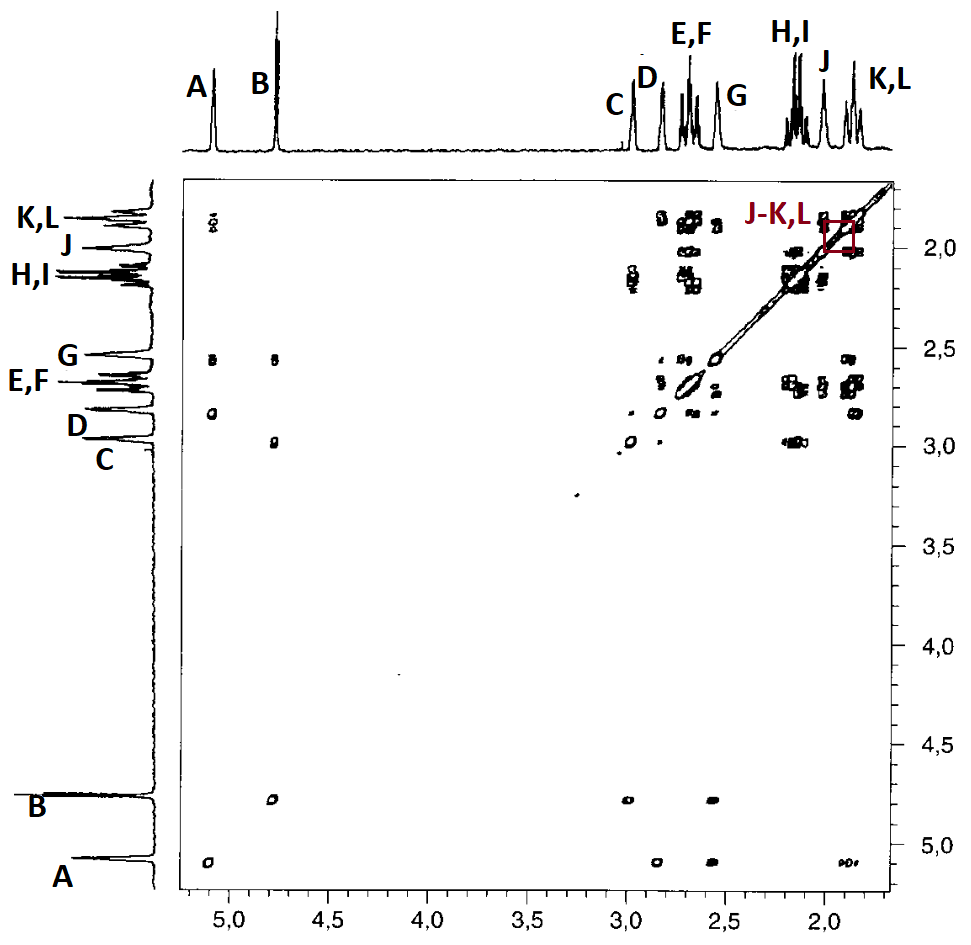
A COSY H/H allows us to get the solution.
The 1H-NMR spectrum was recorded on a spectrometer with a magnetic field of 9.5 Tesla, in which the 1H resonates at 400 MHz. CDCl3 is the solvent although its signal 7.26 ppm is out of the plotted range.
Make first a list of preliminary observations/conclusions:
1) The most deshielded signals must belong to the two CHBr groups.
2) Since two very different CHBr groups are observed (5.09 ppm and 4.75 ppm), molecules 2 and 5 can be easily excluded because their CHBr groups must be isocronous - same chemical shift -by symmetry.
3) Between compounds 3 and 4 the biggest difference is that in 4 the CHBr groups share a CH common neighbor. That doesn't happen in 3.
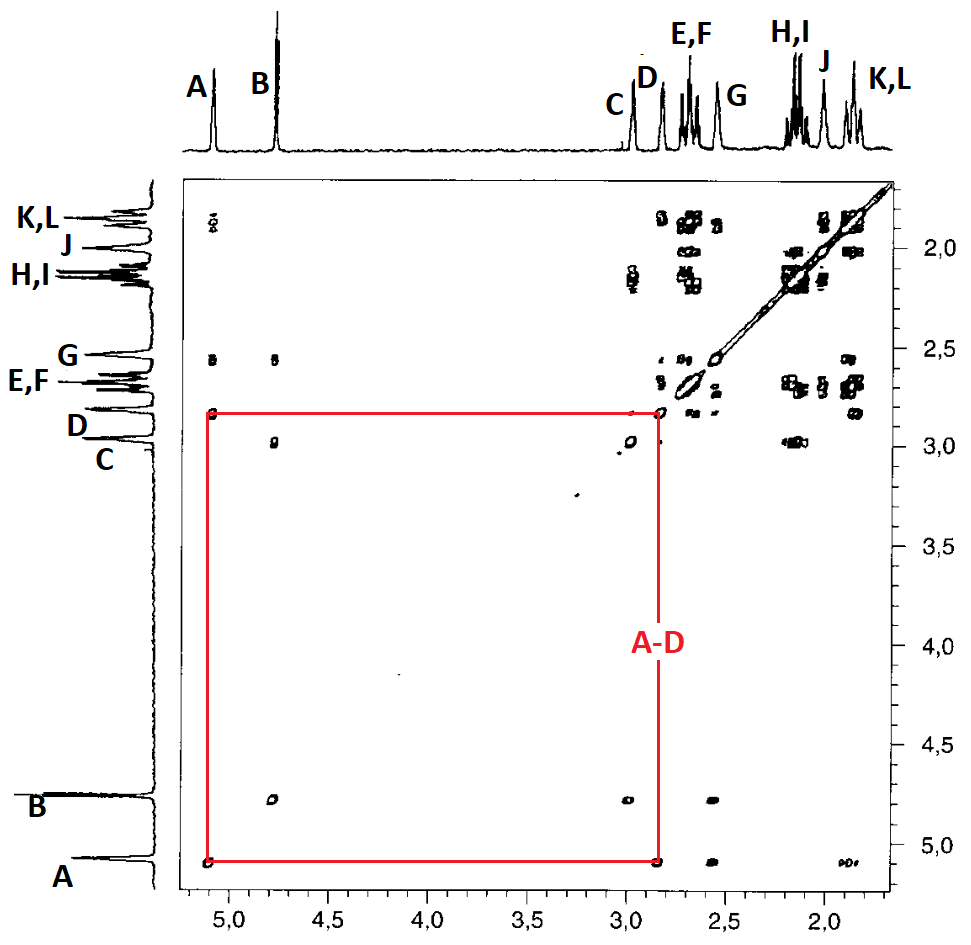
Let's see how the 2D COSY H/H recorded in the very same instrument solves the problem for us.
In the interpretation it is unnecessary to draw the diagonal that is self-evident.
Let's assign a letter to every 1D-spectrum signal.
Remember that some signals displayed a double integral. That's why we have written two letters for each of the three of them.
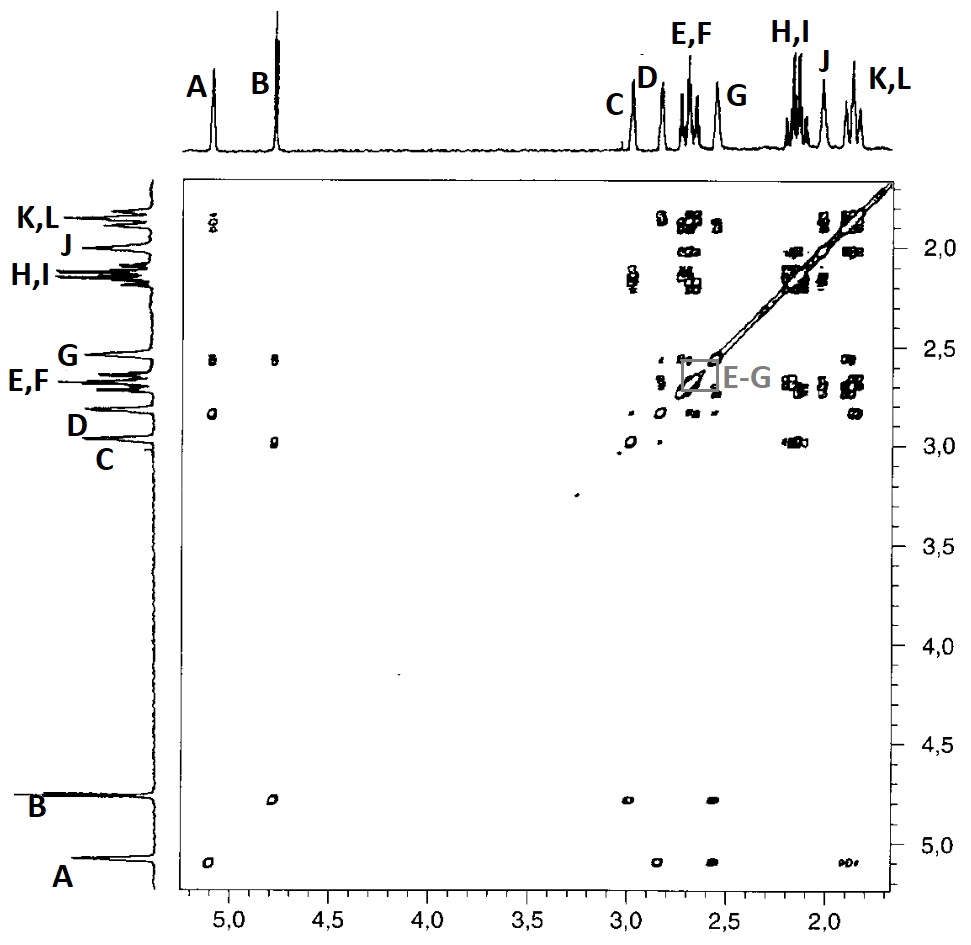
Let's draw all correlations by means of the corresponding paralelograms and take note of the related letters:
Correlations A-G and B-G are key to solve our problems because they show that signals A and B belonging to the two CHBr groups share a common correlation with G.
Therefore, the right structure for the compound is 4.
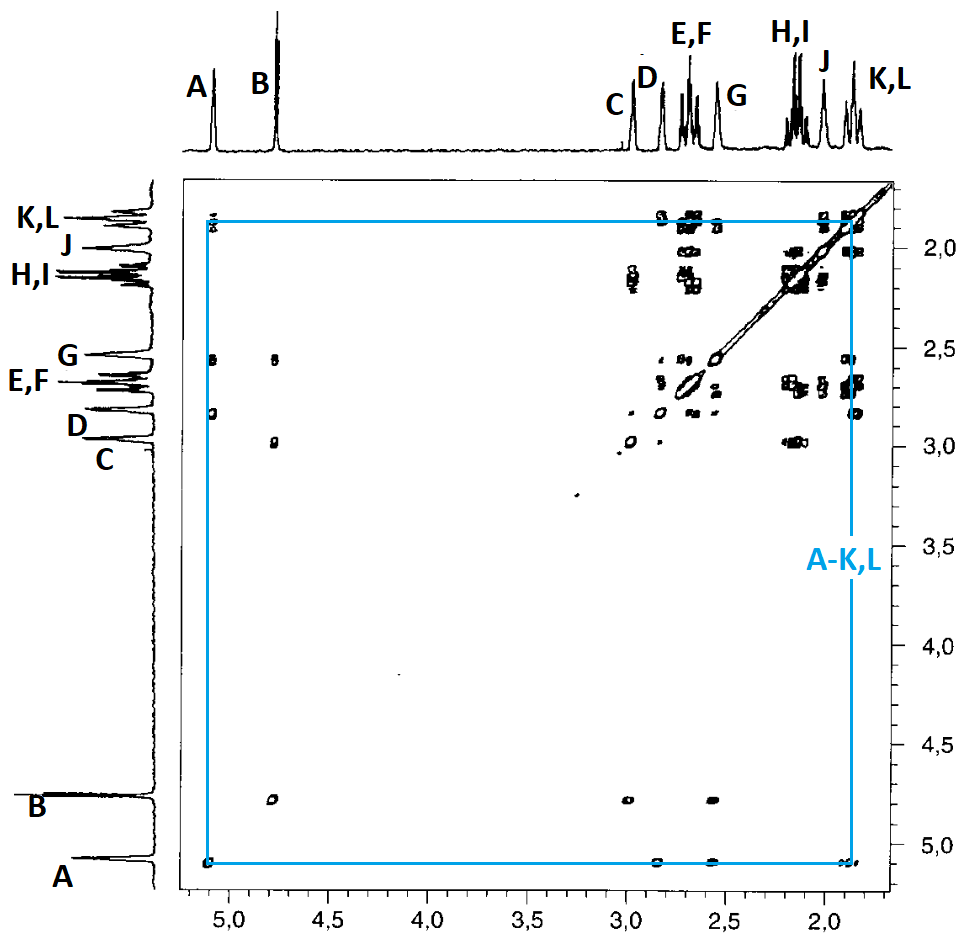
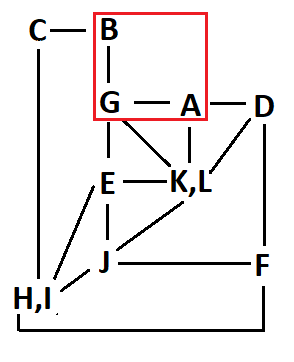
Although the problem is already solved with the inspection of solely two correlations, let's consider the whole bunch of them and see whether they corroborate the structure.
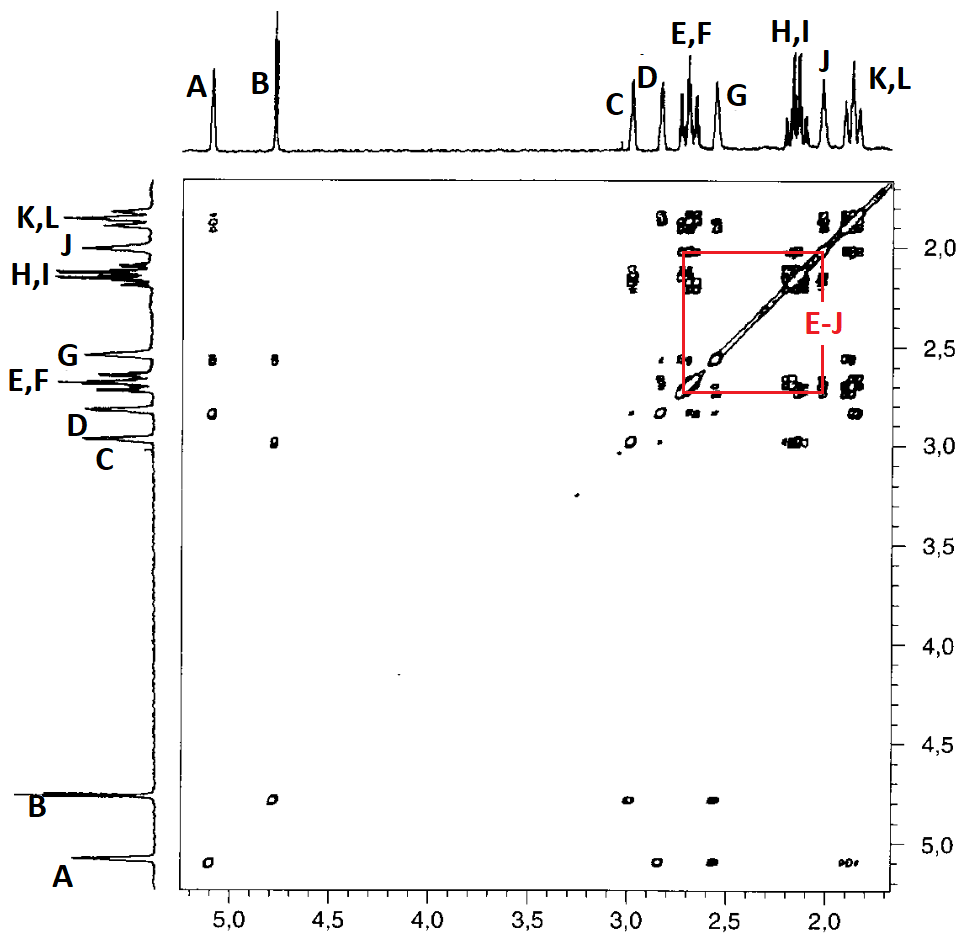
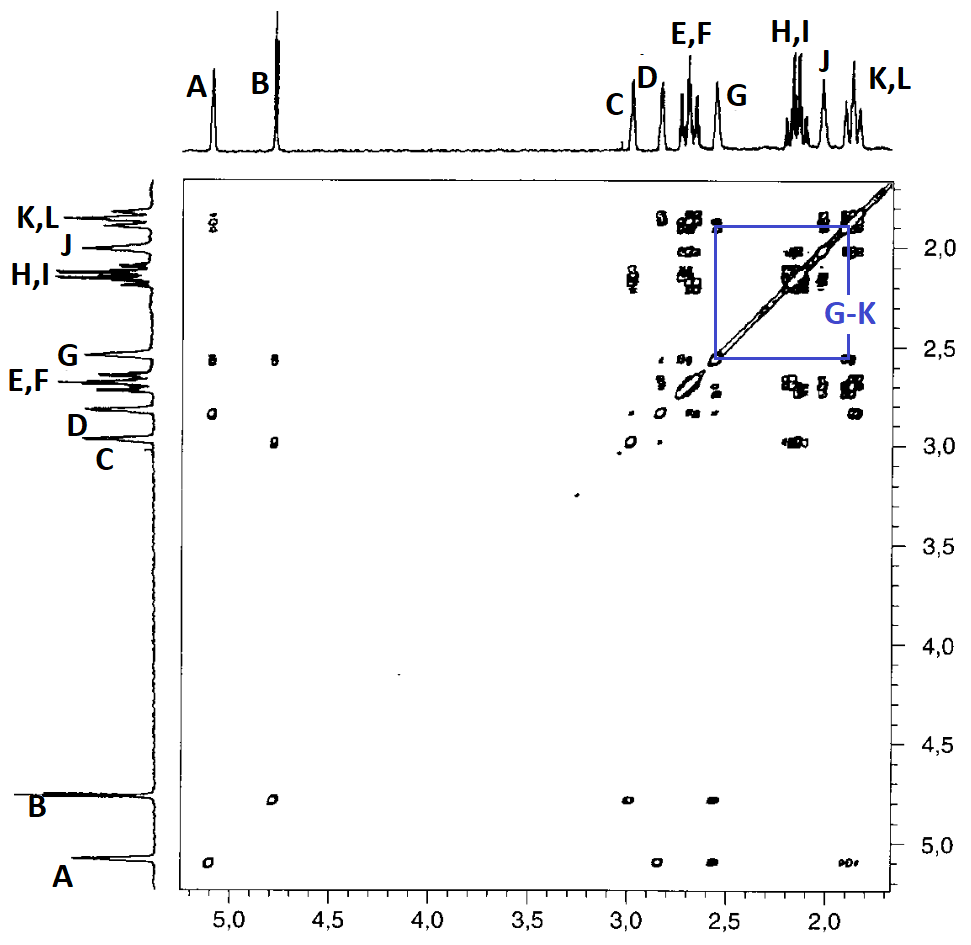
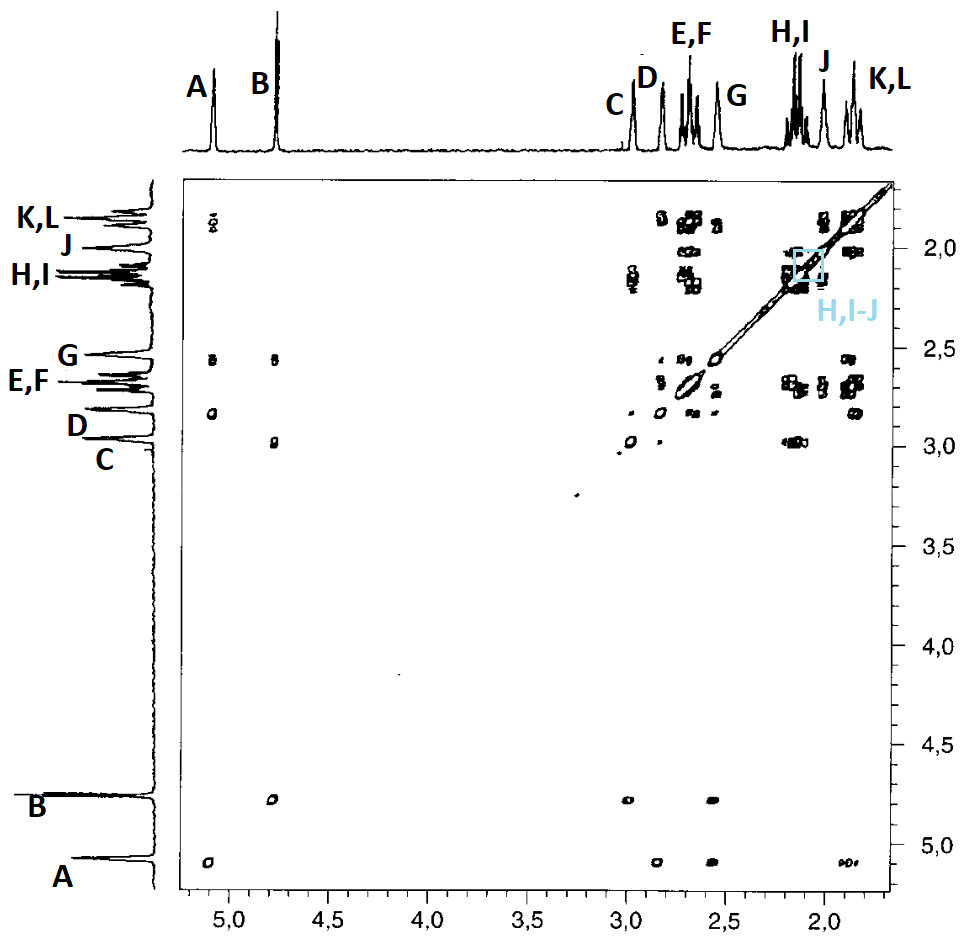
The correlation summary is as follows:
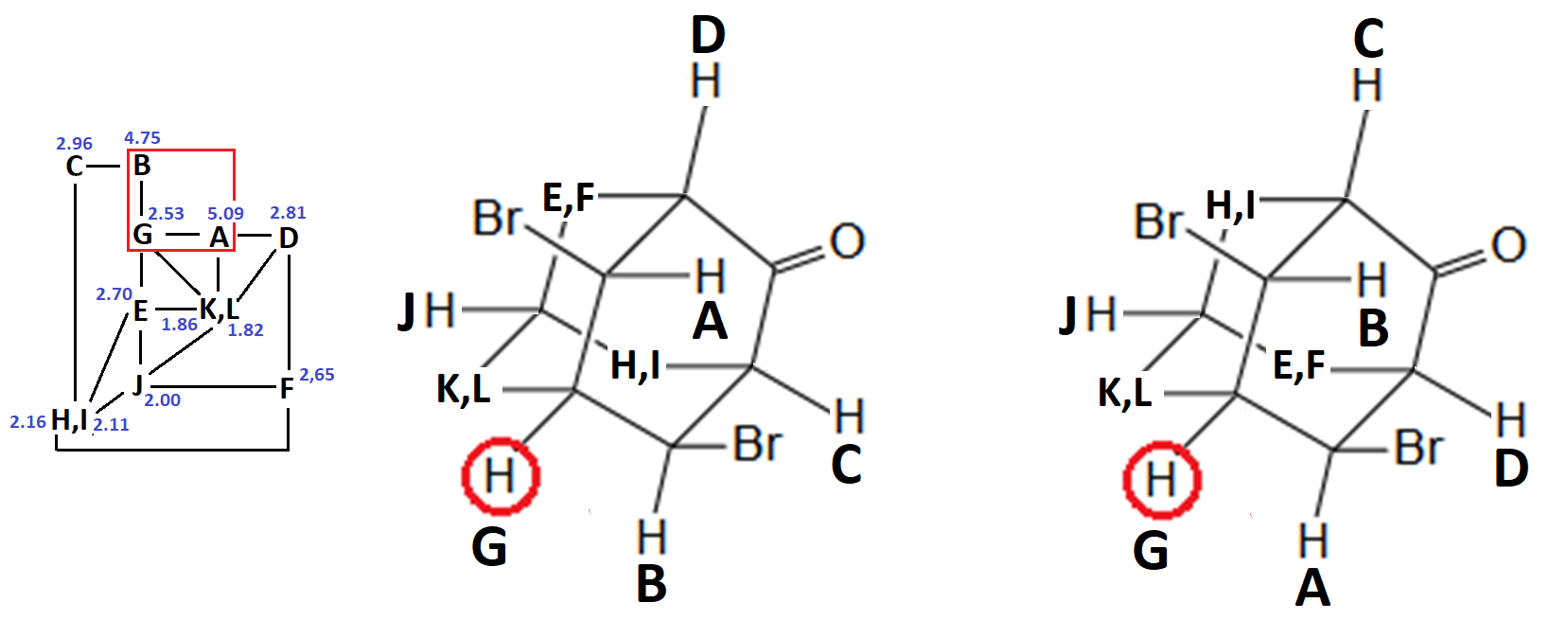
In principle, the correlation map is compatible with two slightly different assignments, both compatible with the structure of compound 4:
Yet, there still are some inconsistencies:
For instance: How come that protons D and L show a correlation when, as so-assigned, they are at a distance of 5 bonds?
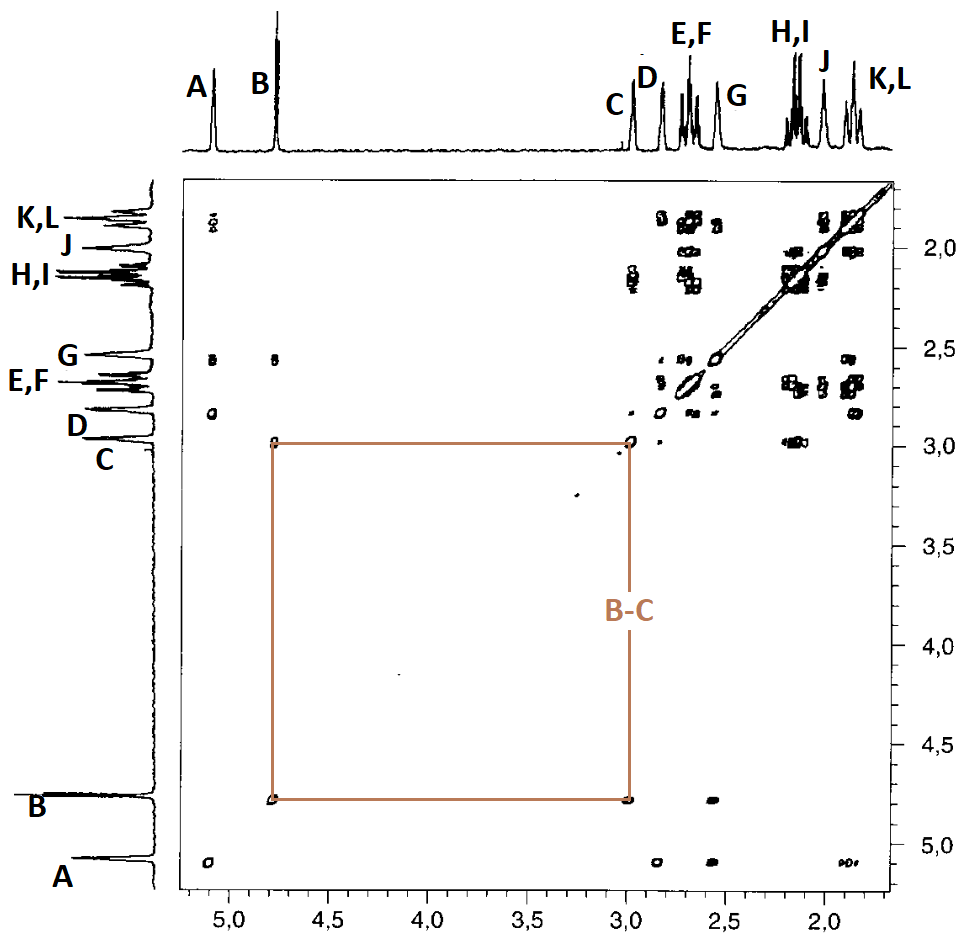
We have clearly overlooked something in the assignment because we assumed too quickly that the pairs E-F, H-I y K-L belong to the indicated CH2.
In cyclohexanes, the axial protons are usually more shielded than the equatorial counterparts by ca. 0.5 ppm.
A more plausible assignment could be the following. Do you agree?